FDA proposes front-of-package nutrition labels to combat chronic diseases
Companies given three to four years to comply with new labeling requirements
2025-01-22
The United States Food and Drug Administration (FDA) has proposed a plan to introduce front-of-package nutrition labels on packaged foods and beverages. This initiative aims to help consumers identify healthier options as part of a broader effort by the U.S. government to combat chronic diseases such as diabetes, heart disease, and cancer, which are leading causes of death and disability in the country.
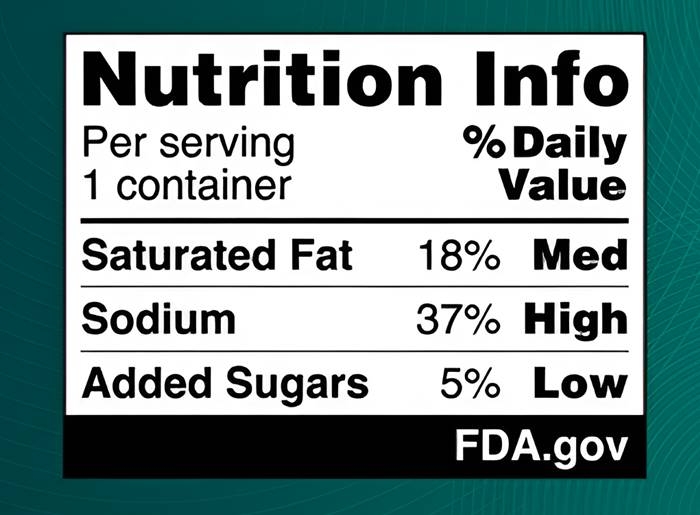
The proposal, announced on January 14, outlines a system that categorizes the levels of saturated fats, sodium, and added sugars in products as "low," "medium," or "high." The label would resemble the existing Nutrition Facts panel found on packaging and would apply to most packaged foods and drinks. If approved, companies with annual sales exceeding $10 million would have three years to comply, while smaller businesses would be granted four years. The public comment period for this proposal is open until May 16.
FDA Commissioner Robert Califf emphasized the importance of this measure, stating that diet-related chronic diseases impact millions of Americans. He noted that the proposal aims to make it easier for consumers to make informed decisions quickly and practically, contributing to improved public health.
The proposal presents logistical challenges for manufacturers. Jennifer Hatcher, a representative of the Food Industry Association (FMI), pointed out that requiring the label to appear in the upper third of the package front would necessitate redesigning many product packages. This redesign could displace essential information, such as expiration dates, and impose significant costs on manufacturers. Hatcher argued that these changes would require substantial investment with potentially limited public health benefits.
Conversely, Peter Lurie, president of the Center for Science in the Public Interest, a U.S.-based nonprofit organization, called the measure "long overdue." Lurie stated that the initiative could influence consumer choices, encourage companies to create healthier products, and reduce preventable diseases like type 2 diabetes and heart conditions. He also suggested that the FDA consider international evidence supporting similar systems already in place in South American countries.
The FDA referenced studies showing that many ultra-processed products in the U.S. contain high levels of saturated fats, sodium, and added sugars, which are linked to the development of various chronic diseases.
Founded in 2007, Vinetur® is a registered trademark of VGSC S.L. with a long history in the wine industry.
VGSC, S.L. with VAT number B70255591 is a spanish company legally registered in the Commercial Register of the city of Santiago de Compostela, with registration number: Bulletin 181, Reference 356049 in Volume 13, Page 107, Section 6, Sheet 45028, Entry 2.
Email: [email protected]
Headquarters and offices located in Vilagarcia de Arousa, Spain.