The mystery of tartaric acid: the wine's diamonds
The essential role of tartaric acid in winemaking

The process of winemaking, a tradition as old as civilization itself, holds within its folds a series of chemical marvels, one of which is the role of tartaric acid, often referred to as the 'diamonds of wine.' This article delves into the science behind tartaric acid, its discovery, and its unique characteristics that make it an integral component of winemaking.
Grape must, the initial step in the journey from grape to wine, is a complex mixture. It primarily consists of water, accounting for 70 to 80% of its content. The sweetness of the must comes from sugars like glucose and fructose, essential for fermentation. Polyphenols, found in the skins and seeds of grapes, contribute to the wine's beauty and anti-aging properties. The must also contains pectic substances, nitrogen compounds, minerals, enzymes, and vitamins. However, the focus of this discourse is on the organic acids present in the must, particularly tartaric acid.
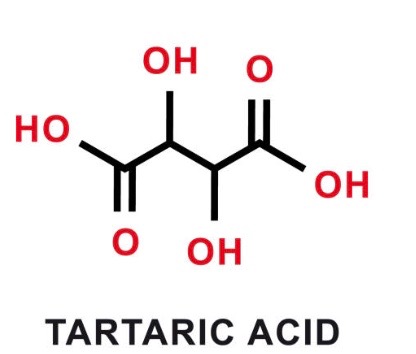
Tartaric acid, the most abundant acid found in grapes, plays a pivotal role in winemaking. In its isolated form, it is a white powder with the chemical formula C4H6O6. This acid is crucial in regulating the wine's characteristics. By dissolving in wine and lowering its pH, tartaric acid increases the wine's acidity. This adjustment serves several purposes: it prevents bacterial growth during malolactic fermentation and imparts a refreshing acidity to the wine. Yet, an excess of tartaric acid can lead to the formation of crystals in the cork or at the bottom of the bottle, known as tartar crystals or 'the diamonds of wine.'
The Discovery of Tartaric Acid
The journey to understanding tartaric acid began in 1769 with Carl Wilhelm Scheele, who first isolated it. The real breakthrough, however, came with Louis Pasteur. Investigating the nature of tartaric acid, Pasteur discovered that the acid derived from wine grapes and its laboratory-synthesized counterpart had differing properties in polarizing light, despite having the same chemical composition. Pasteur's meticulous examination under a microscope revealed two types of crystals forming from a tartaric acid salt, each being a mirror image of the other. When he separated these crystals and dissolved them in water, he observed that they rotated polarized light in opposite directions, one clockwise and the other counterclockwise. This led to the discovery of enantiomers – molecules that are mirror images of each other but cannot be superimposed.

Pasteur's discovery highlighted the concept of chirality – the property of an object (or a molecule) being non-superimposable on its mirror image, akin to the relationship between left and right hands or a pair of shoes. This concept is not only vital in chemistry but also has significant implications in pharmaceuticals, where certain anti-inflammatory drugs exhibit chirality. In nature, like in grapes, only one enantiomer is usually present. In contrast, synthesizing tartaric acid in the laboratory results in a 50-50 mixture of both enantiomers, leading to the non-polarization of light.
A Toast to Tartaric Acid
The article concludes with a nod to an ancient Roman custom where toast (literally, toasted bread) was added to wine. The charcoal formed from toasting bread helped reduce the excess tartaric acid in the wine, improving its quality. This practice is whimsically echoed in the English phrase "to make a toast," linking the culinary world of bread to the ceremonial world of wine.
Founded in 2007, Vinetur® is a registered trademark of VGSC S.L. with a long history in the wine industry.
VGSC, S.L. with VAT number B70255591 is a spanish company legally registered in the Commercial Register of the city of Santiago de Compostela, with registration number: Bulletin 181, Reference 356049 in Volume 13, Page 107, Section 6, Sheet 45028, Entry 2.
Email: [email protected]
Headquarters and offices located in Vilagarcia de Arousa, Spain.